Date: September 26-29.2025 | Booth:4.1H-4.1P08
Address: Building 2, No.72 Shengli Road, Yixiu, Anqing, Anhui, China.
- Email: info@listerbiomed.com
- Phone: +86 556 5689070
News&Media
In sterilization program validation, although the sterilization effect can be evaluated by monitoring certain parameters of the sterilization process, the degree of sterilization of biological indicators is the most intuitive indicator for evaluating the effectiveness of a sterilization program.
A biological indicator incubator is a constant-temperature cultivation device specifically designed to culture biological indicators that have undergone sterilization processes, verifying whether the sterilization meets standard requirements.
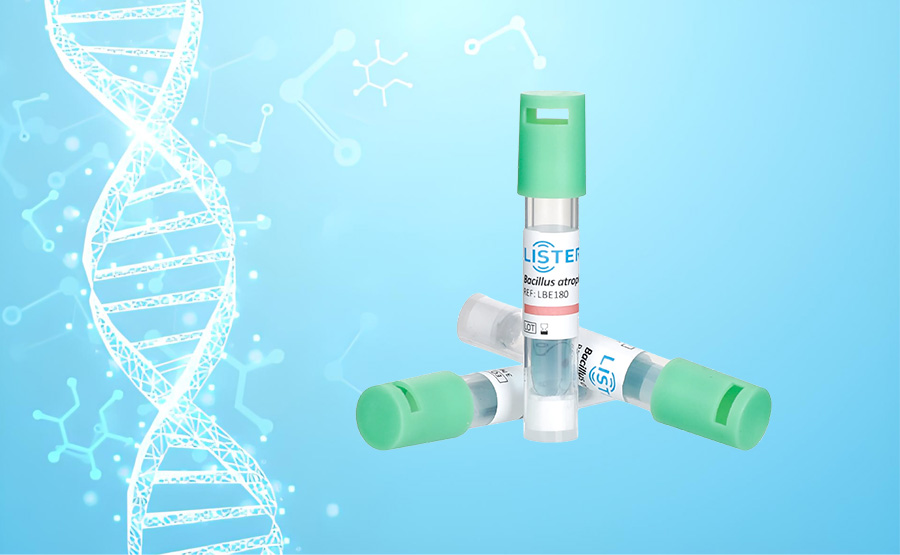
Biological indicators (BIs) are specialized live microbial products used to verify the performance of sterilization equipment, validate sterilization procedures, and monitor the effectiveness of sterilization during production processes.
Biological indicators (BIs) play a crucial role in ensuring sterility and patient safety in the medical field. These specialized tools contain highly resistant bacterial spores that provide definitive proof of sterilization effectiveness. Hospitals, pharmaceutical companies, and medical device manufacturers rely on BIs to validate sterilization processes and comply with stringent regulatory standards.
The LBE180 Rapid Biological Indicator, when used with an automatic reader (incubation temperature 35-39°C), provides a fluorescence test result in a short period (LBE180-180 minutes) with an accuracy of ≥97%.
Biological indicators (BIs) provide the highest assurance of autoclave sterilization effectiveness by using live bacterial spores (Geobacillus stearothermophilus) as a direct challenge.
LISTER BIOMEDICAL CO.,LTD was invited to participate XIII Congreso Panamericano de Esterilización Hospitalaria FELACEH - SOCIENEE 2025..Introduce the principles and methods of reprocessing reusable medical devices. With the continuous updating and iteration of medical devices, introduce the limitations and risks of existing methods, and analyze effective solutions for the future.