01 Jan, 2026
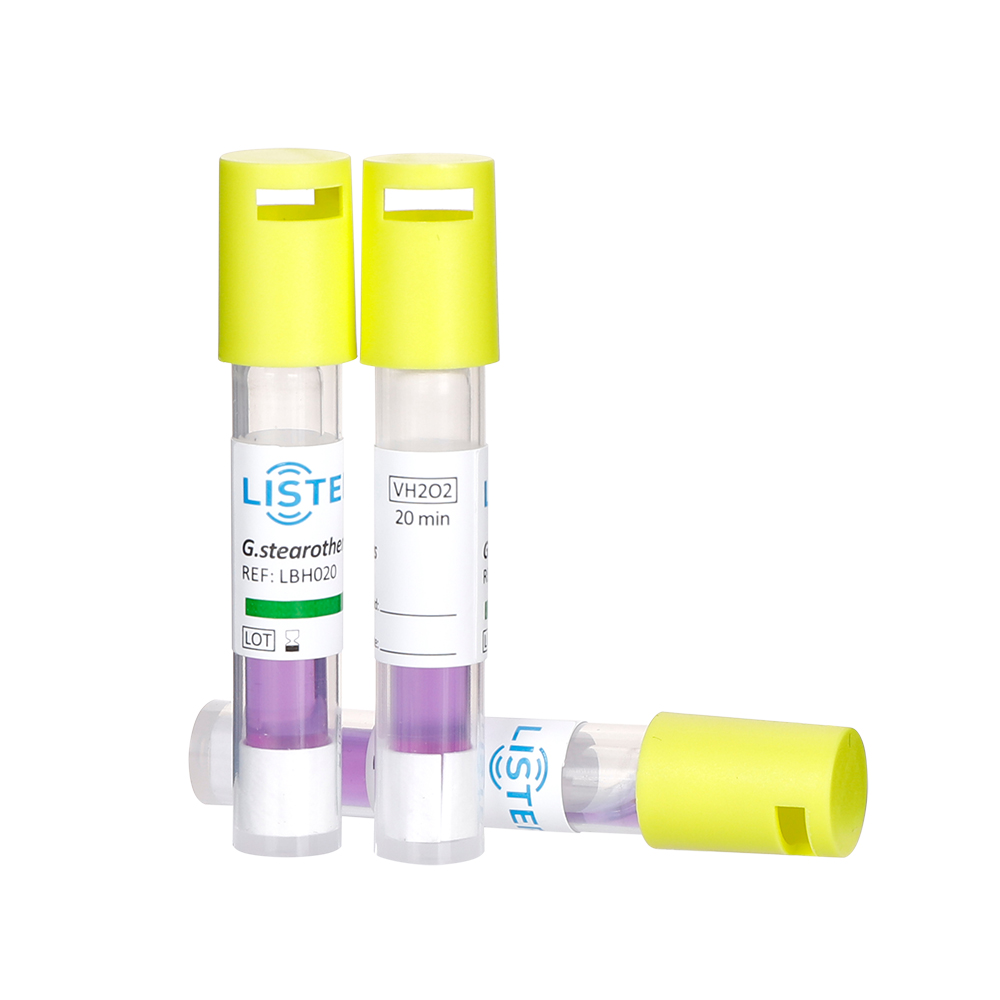
Biological indicators (BIs) provide the highest assurance of autoclave sterilization effectiveness by using live bacterial spores (Geobacillus stearothermophilus) as a direct challenge.
Key Steps:
1.select & Place:
· Use BIs (self-contained vials or spore strips with separate medium).
· Place them in the autoclave's coldest spots (e.g., center of dense packs, bottom front near drain, inside lumens if validated) to test the hardest-to-sterilize areas. Use multiple BIs for complex loads.
2.Run Cycle: Process the load with the BI(s) using the correct, validated cycle parameters.
3.Retrieve & Activate:
· Let load and BI cool.
· For self-contained BIs: Crush the inner ampoule to mix spores and medium. For strips: Aseptically transfer to growth medium.

4.Incubate:
· Label BIs clearly.
· Incubate at the specified temperature (usually 55-60°C) for the required time (often 24-48 hrs rapid, 7 days conventional).
· CRITICAL: Include an unprocessed Positive Control (proves spores/media work) and an uninoculated Negative Control (proves media sterility).
5.Read Results:
· PASS (Sterile): Processed BI shows NO change (e.g., remains purple/clear). Positive Control shows change (yellow/turbid); Negative Control shows no change.
· FAIL (Not Sterile): Processed BI SHOWS change (yellow/turbid). Immediately: Take autoclave out of service, quarantine the load, and investigate.
6.Document: Record autoclave ID, cycle details, BI lot, placement, incubation, results (including controls), and technician.
LISTER is committed to Present Scientific BI + CI + PCD Integrated Solutions for Sterilization. Our customers span across Europe, the Middle East, South America, and Southeast Asia, and in the future, more people will use and recognize our biological idncators.
Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs), Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.

Tags: