01 Jan, 2026
(I) Sterilization Efficacy Monitoring for LTVH₂O₂ Sterilizers:
Regulatory Basis: Sterilization efficacy monitoring should be conducted in accordance with WS310.3 "Central Sterile Supply Department (CSSD) - Part 3: Surveillance Standard for Cleaning, Disinfection and Sterilization" and the guidance provided in the sterilizer user manual. Monitoring Content: Physical monitoring, chemical monitoring, and biological monitoring.
1. Physical Monitoring
1.1 For each sterilization cycle, critical parameters (e.g., chamber pressure, temperature, plasma generator output power, sterilization time) must be continuously monitored and recorded. Sterilization parameters must comply with the specifications in the sterilizer user manual.
1.2 If the sterilizer is capable of monitoring hydrogen peroxide concentration, the concentration monitored should be the actual effective concentration during the sterilization process. The product concentration of the sterilant (53%-60%) must not be used as a substitute.
1.3 If physical monitoring fails, the entire batch of sterilized items must not be released and should be reprocessed. A comprehensive investigation must be conducted, including equipment performance, loading method, packaging materials, etc. If the failure is related to a critical component malfunction, timely repair is required. After troubleshooting, the sterilizer must undergo three consecutive cycles of physical monitoring, chemical monitoring, and biological monitoring for all sterilization programs in use. The sterilizer can only be used again after all monitoring results are acceptable.

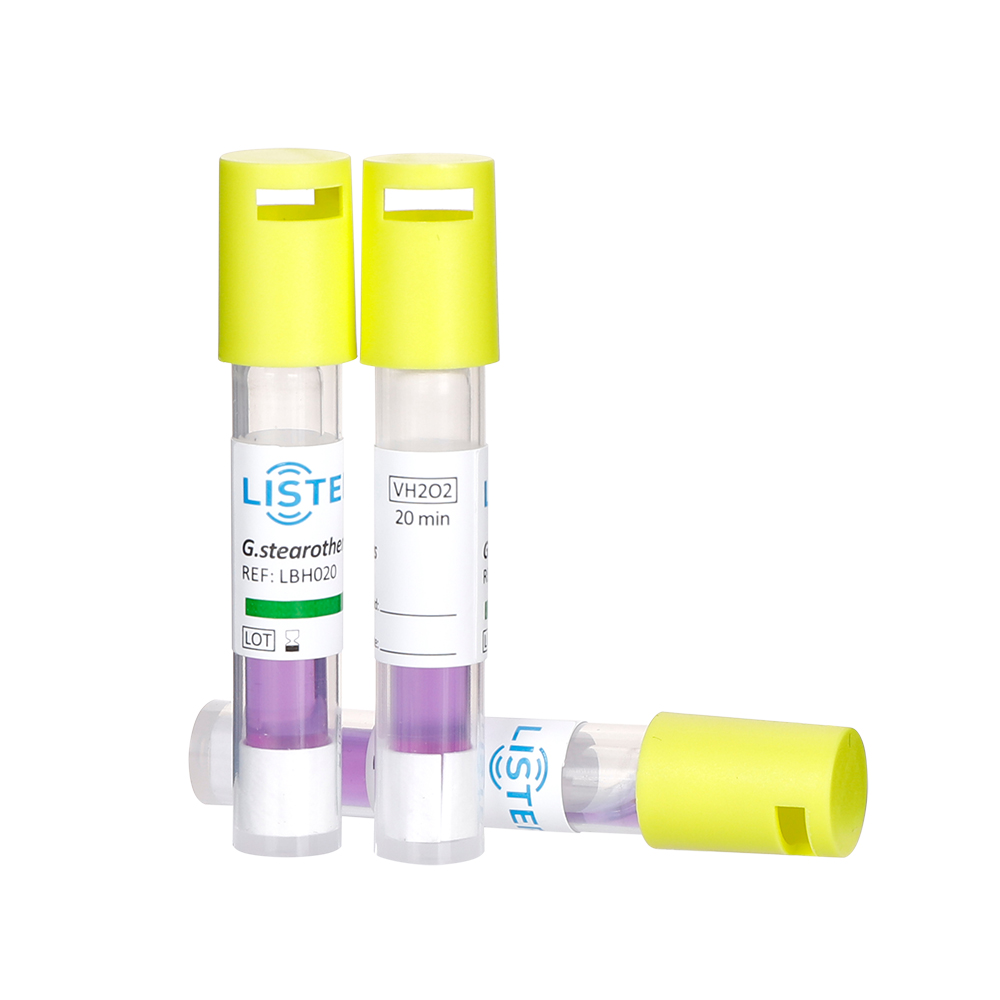
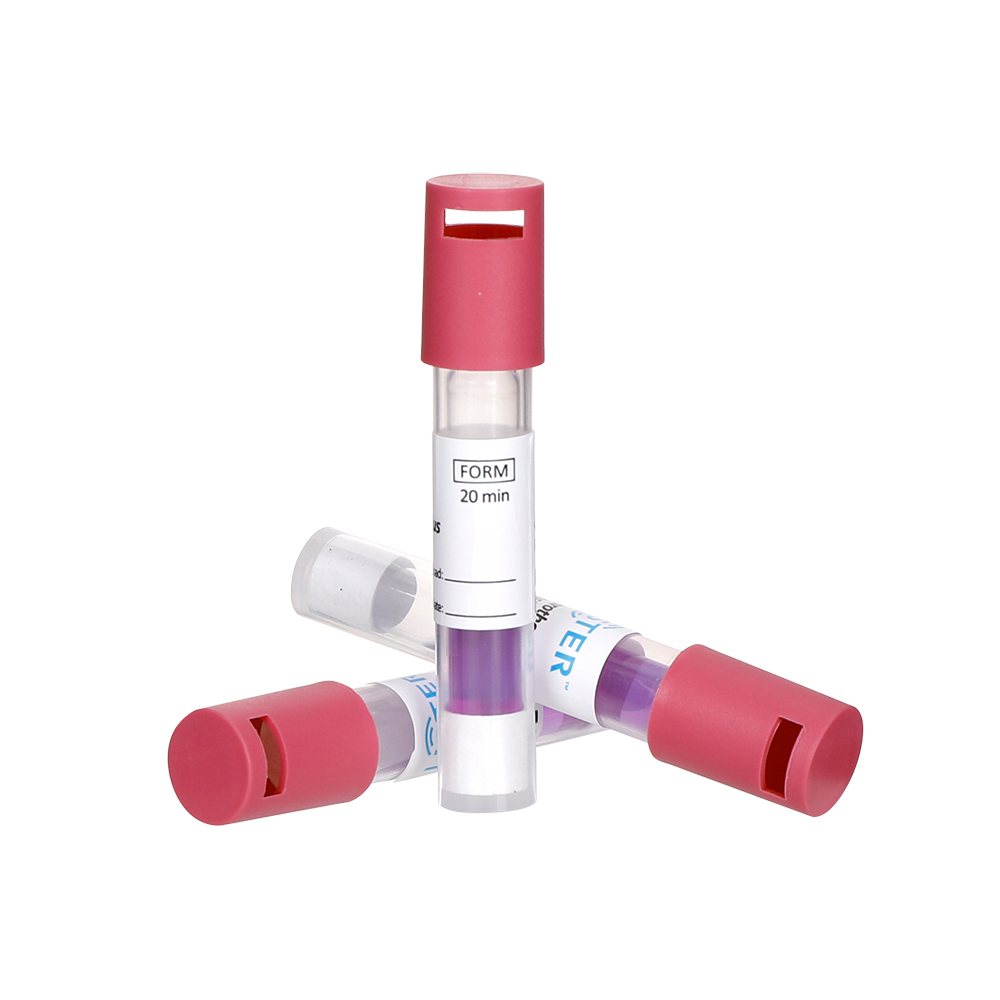
2.1 An external chemical indicator (CI) specific for LTVH₂O₂ sterilization must be used on the outside of each package as an indicator of the sterilization process.
2.2 An internal chemical indicator specific for LTVH₂O₂ sterilization must be placed at the site within each package deemed most difficult to sterilize. Its color change should be observed to determine if sterilization requirements were met. Class 1 CIs (process indicators) are not recommended for use as internal indicators.
2.3 The results of both external and internal chemical indicators should be interpreted according to the manufacturer's instructions for the specific indicator product.
2.4 Items with a failing external CI must not be released. Items with a failing internal CI must not be used. The cause of the failure must be analyzed, and corrective actions taken until monitoring results meet requirements.
3.1 Biological monitoring should be performed at least once per day for each active sterilization program in use. Frequency may be increased based on the healthcare facility's management requirements. Biological indicator test packs should be prepared according to the requirements of Appendix in WS310.3.
3.2 When sterilizing non-lumened instruments, a non-lumened biological indicator test pack should be used. The test pack should be placed in the location within the sterilizer chamber identified as most difficult to sterilize, as specified in the sterilizer user manual.
3.3 When sterilizing lumened instruments, a Process Challenge Device (PCD) with a lumen should be used for monitoring. The lumened biological PCD should be placed in the location within the sterilizer chamber identified as most difficult to sterilize, as specified in the sterilizer user manual. The model and specifications of the lumened PCD must comply with the requirements of the instrument manufacturer.
3.4 If biological monitoring fails, all sterilized items processed since the last acceptable biological monitoring result that have not yet been used should be recalled as soon as possible and reprocessed. The cause of the failure must be analyzed and corrective actions implemented. The sterilizer can only be used again after achieving three consecutive acceptable biological monitoring results.
(II) Routine Maintenance
1. Daily maintenance of the sterilizer should be correctly performed according to established operating procedures.
2. The sterilizer should remain powered on (in standby mode).
3. Indicators related to sterilizer operation should be checked to ensure normal display.
4. The functionality of foot or hand control switches should be checked.
5. The exterior and chamber interior of the sterilizer should be kept clean.
6. Regular Maintenance and Periodic Testing
Tags: