01 Jan, 2026
In sterilization and disinfection processes, ensuring effectiveness is non-negotiable. Whether in healthcare, pharmaceuticals, or laboratory settings, professionals rely on indicators to validate that sterilization conditions are met. Two primary tools for this purpose are biological indicators (BIs) and chemical indicators (CIs). While both serve critical roles in quality assurance, they differ fundamentally in design, function, and reliability. Let’s explore their key distinctions to help you choose the right tool for your needs.
What Are Biological Indicators?
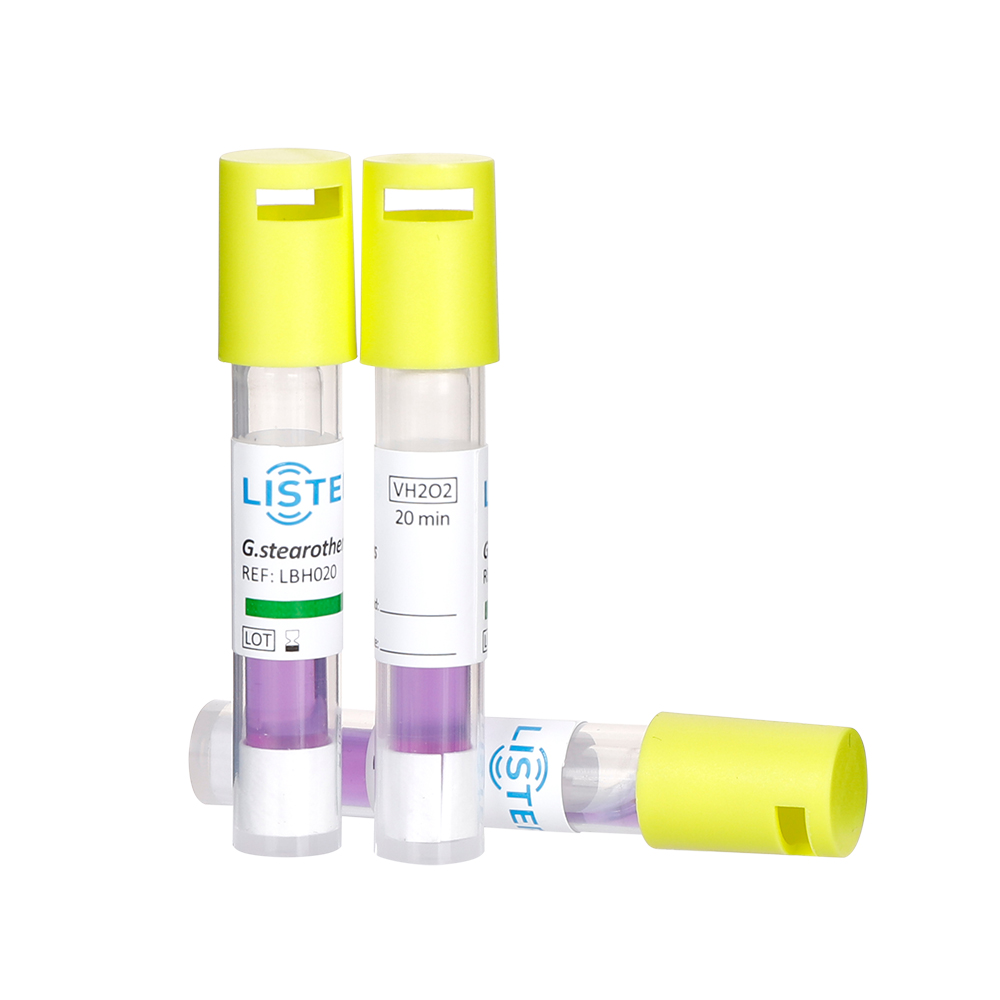
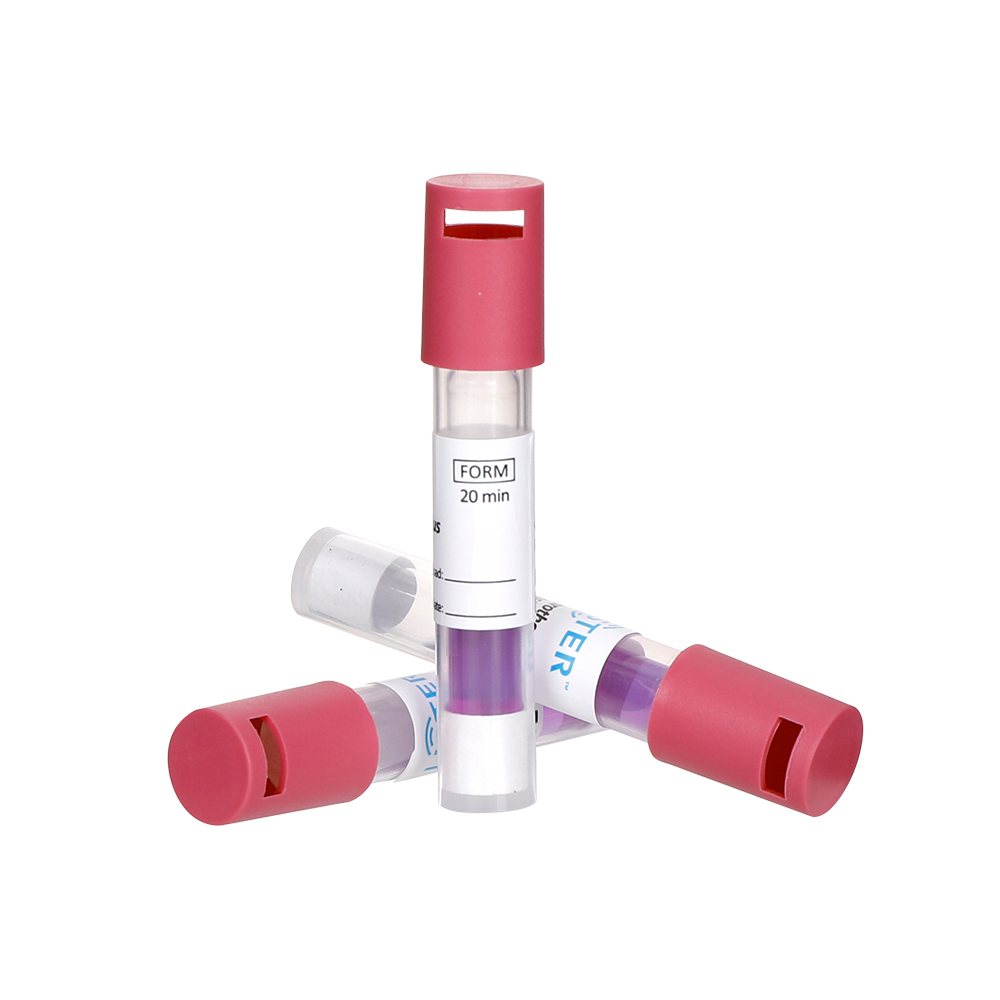
Biological indicators are living microorganisms—typically highly resistant bacterial spores (e.g., Geobacillus stearothermophilus for steam sterilization)—used to directly assess the lethality of a sterilization process. These spores are placed in a carrier material (e.g., strips, vials, or discs) and exposed to the sterilization cycle. After processing, the BI is incubated under controlled conditions. If no microbial growth occurs, the sterilization cycle is deemed effective.
Key Features of BIs:
Live Testing: Provide a direct measure of sterilization efficacy by challenging the process with the most resistant organisms.
Definitive Results: A "pass" or "fail" outcome confirms whether microbial life has been eliminated.
Regulatory Reliance: Required for validating sterilization cycles in high-risk applications (e.g., surgical instruments, implants).
What Are Chemical Indicators?
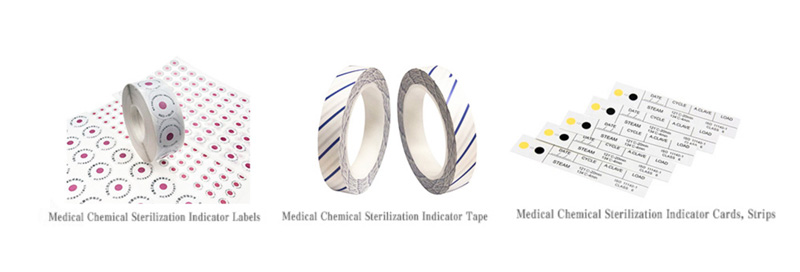
Chemical indicators use sensitive chemicals that undergo visible physical or chemical changes (e.g., color shifts, melting) when exposed to specific sterilization conditions, such as temperature, time, or vapor concentration. Common examples include autoclave tape, indicator strips, or labels on packaging.
Key Features of CIs:
Immediate Feedback: Offer real-time visual confirmation that a sterilization parameter (e.g., temperature) was reached.
Process-Specific: Different CIs monitor distinct variables (e.g., Class 1 indicators confirm exposure, while Class 5 indicators integrate multiple parameters).
Supplemental Use: Serve as a quick check but do not confirm sterilization efficacy alone.
Critical Differences at a Glance
| Aspect | Biological Indicators | Chemical Indicators |
| Test Focus | Directly kills live microbes | Detects physical/chemical conditions |
| Result Type | Definitive (growth/no growth) | Presumptive (pass/fail based on change) |
| Response Time | Hours to days (incubation required) | Instantaneous |
| Accuracy | Gold standard for efficacy validation | Limited to parameter detection |
| Regulatory Role | Mandatory for cycle validation | Used for routine monitoring |
When to Use Each Indicator
Biological Indicators are essential for:
Validating new sterilization equipment or cycles.
Periodic requalification of existing processes (e.g., monthly testing).
High-risk applications where sterility is critical (e.g., surgical tools).
Chemical Indicators are ideal for:
Daily monitoring of sterilization cycles.
Identifying equipment malfunctions or procedural errors.
Providing immediate visual assurance (e.g., color-changing autoclave tape).
Why Both Matter
While biological indicators are the ultimate test of sterilization success, chemical indicators act as the first line of defense. For example, a CI on a surgical instrument package may signal that it underwent a sterilization cycle, but only a BI can confirm that microbes were eradicated. Together, they create a layered safety net, ensuring compliance with standards like ISO 11138 (BIs) and ISO 11140 (CIs).
Conclusion
Choosing between biological and chemical indicators isn’t an either/or decision—it’s about using them strategically. BIs provide irrefutable evidence of sterility, while CIs offer efficiency and immediacy. By integrating both into your quality control protocols, you ensure patient safety, regulatory compliance, and operational confidence.
LISTER is committed to Present Scientific BI + CI + PCD Integrated Solutions for Sterilization. Our customers span across Europe, the Middle East, South America, and Southeast Asia, and in the future, more people will use and recognize our biological idncators.
Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs), Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.
Tags: