02 Jul, 2025
In hospitals, where infection control is paramount, biological indicators (BIs) serve as critical tools to validate the effectiveness of sterilization processes. Steam sterilization, used for surgical instruments, implants, and other medical devices, must consistently achieve sterility to prevent healthcare-associated infections (HAIs). While physical and chemical monitoring systems provide immediate feedback, biological indicators offer the most reliable proof that sterilization conditions are lethal to even the most resistant microorganisms.
The Role of BIs in Hospital Sterilization
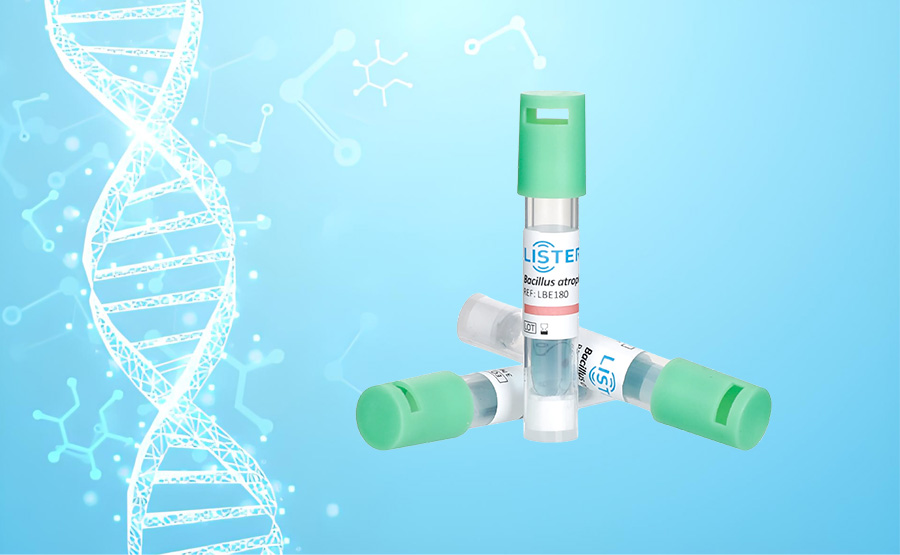
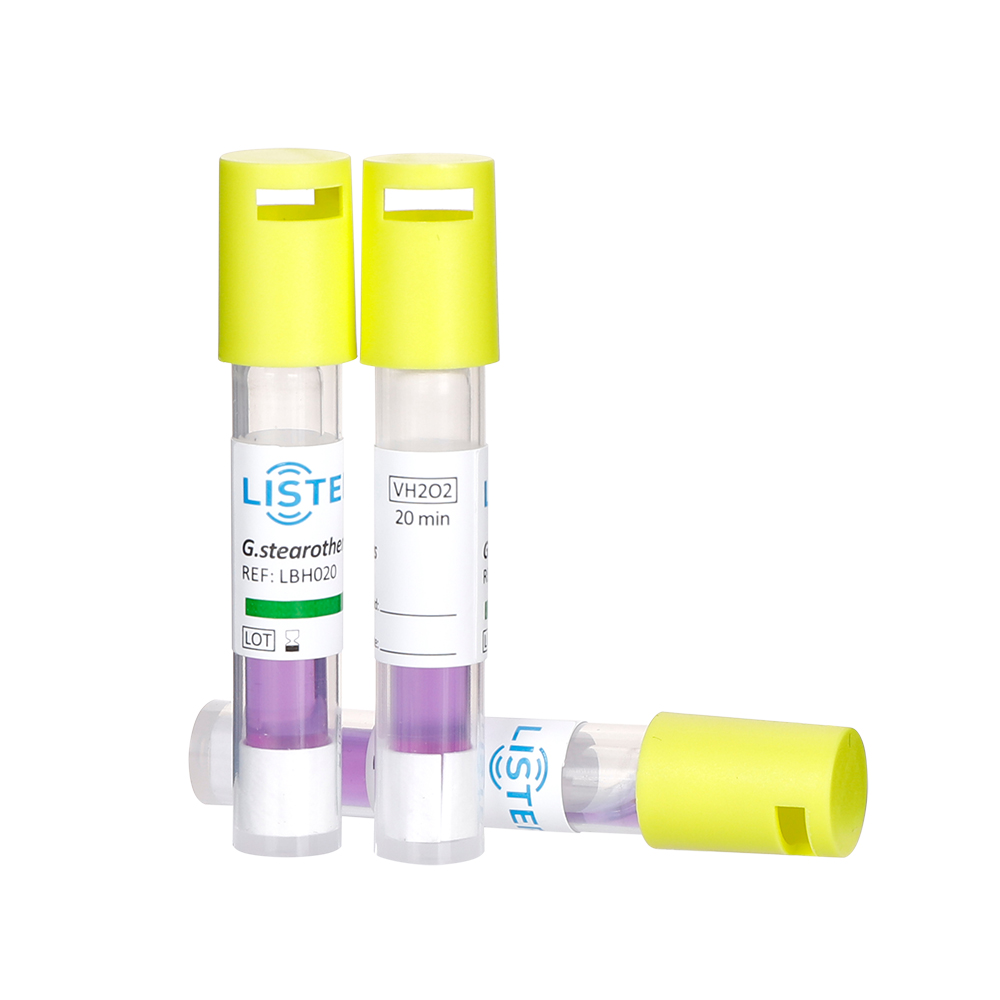
Hospitals rely on BIs to confirm that autoclaves and other sterilizers function correctly under real-world conditions. These indicators contain bacterial spores—typically Geobacillus stearothermophilus for steam sterilization—chosen for their high resistance to heat and moisture. By placing BIs in the most challenging locations (e.g., inside instrument lumens or dense surgical packs), hospitals simulate "worst-case" sterilization scenarios. After a cycle, BIs are incubated to detect any surviving spores. A negative growth result confirms sterility, while positive results trigger immediate corrective actions, such as equipment maintenance or reprocessing.
Future Directions
Advancements in BI technology, such as DNA-based spore detection and wireless monitoring, promise faster, more accurate results. Additionally, integrating BIs with hospital data systems could enable real-time sterility assurance and predictive maintenance of sterilizers.
Conclusion
Biological indicators are indispensable in hospitals, bridging the gap between sterilization theory and practice. By rigorously validating autoclave performance, they protect patients from preventable infections and uphold trust in healthcare systems. As sterilization demands grow more complex, BIs will remain a cornerstone of hospital safety protocols.
LISTER is committed to Present Scientific BI + CI + PCD Integrated Solutions for Sterilization. Our customers span across Europe, the Middle East, South America, and Southeast Asia, and in the future, more people will use and recognize our biological idncators.
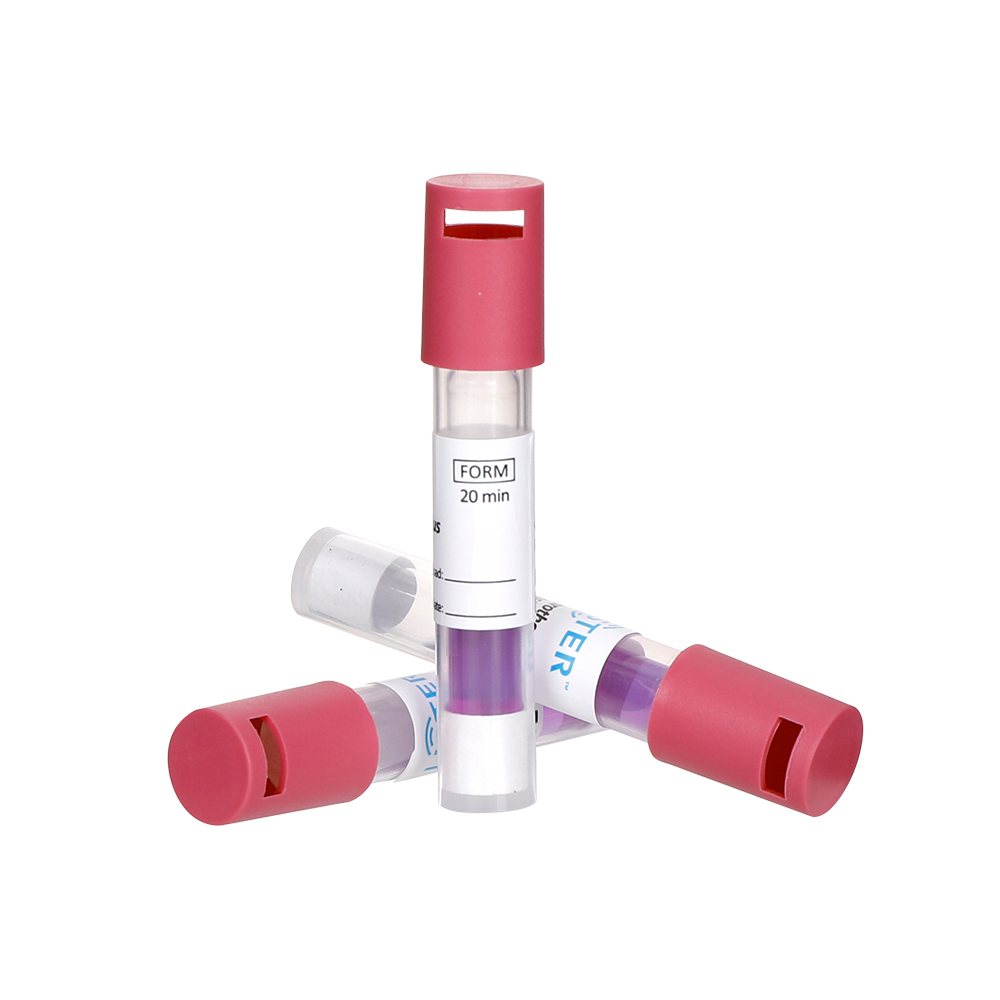
Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs), Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.
Tags: