01 Jan, 2026
How Microbial Spores Ensure Medical Equipment Safety
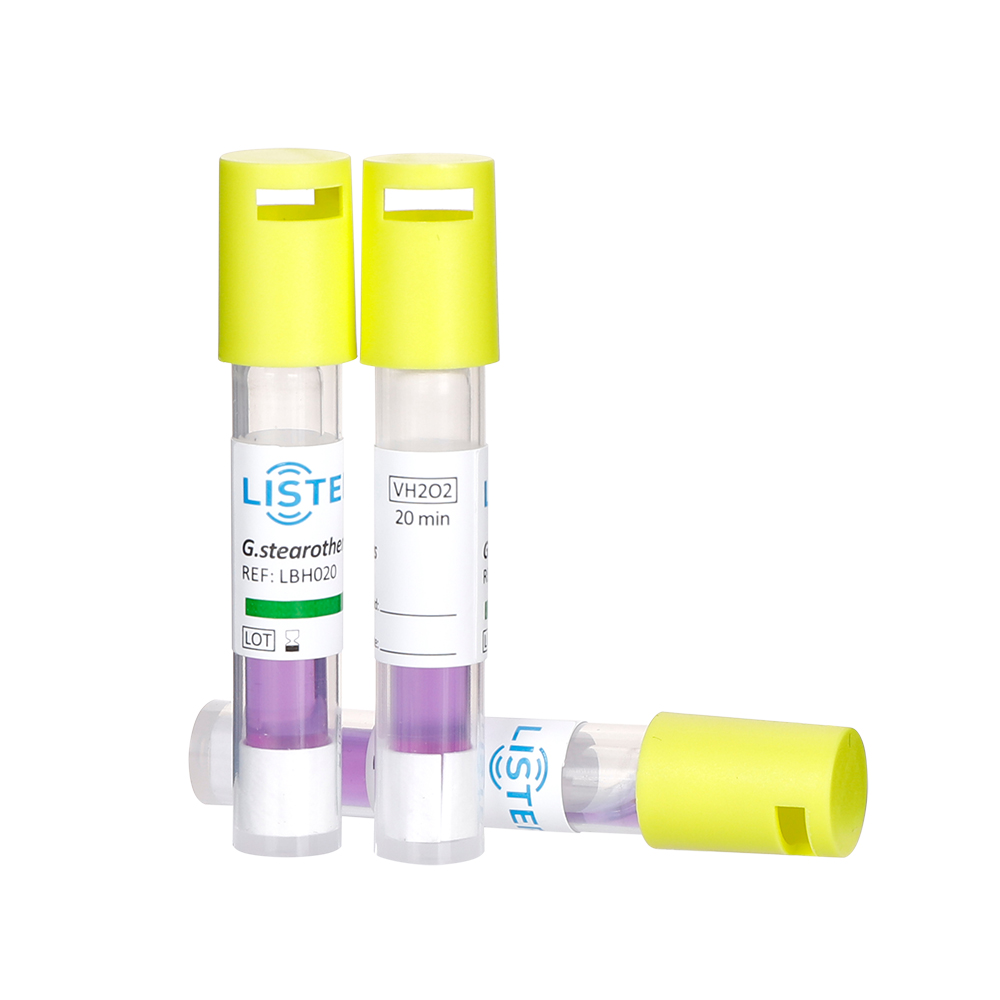
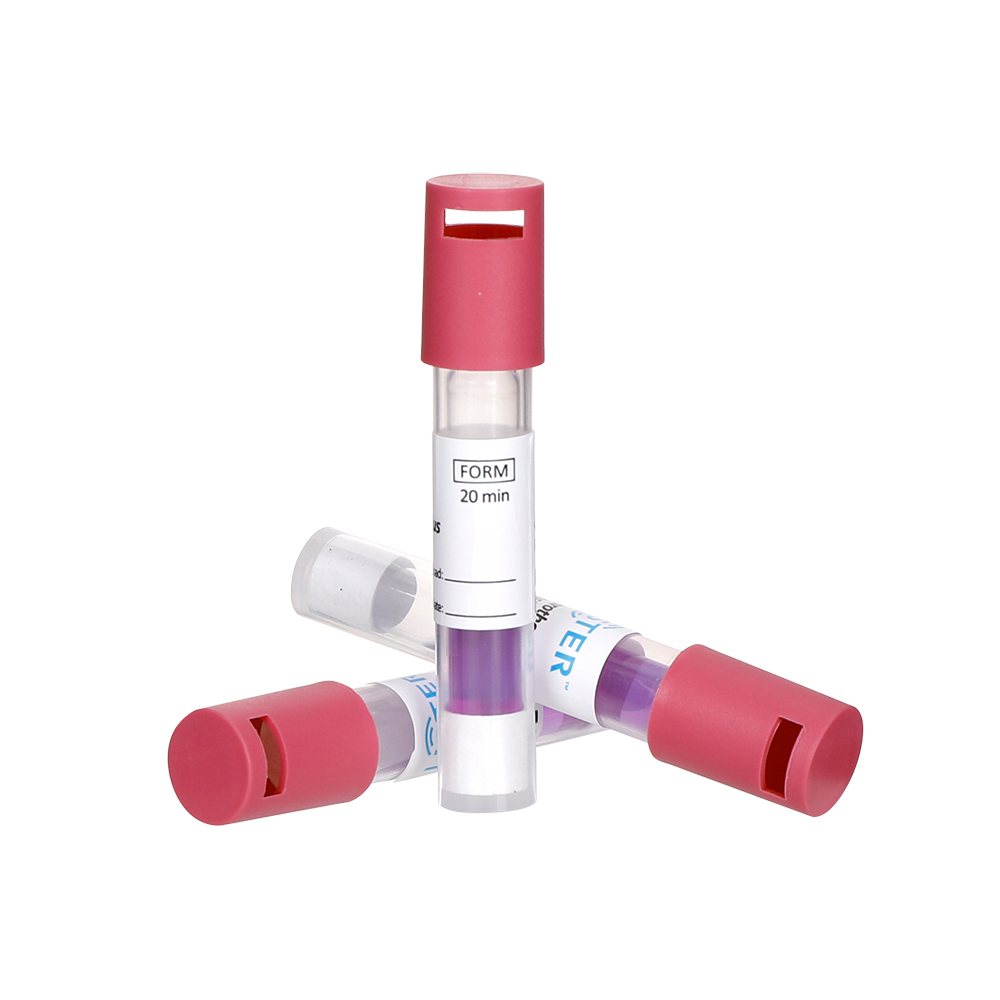
Hospitals and pharmaceutical companies rely on biological indicators to validate sterilization processes. These specialized tools, often containing heat-resistant bacterial spores like Geobacillus stearothermophilus, confirm whether autoclaves or ethylene oxide systems effectively kill pathogens.

How It Works:
Test Strips: Loaded with spores are placed inside sterilization equipment.
Incubation: Post-sterilization, strips are cultured; no growth success.
Compliance: Mandatory for FDA/ISO certification of surgical tools and drug manufacturing.
With rising antibiotic-resistant superbugs, the precision of bioindicators is critical. New rapid-readout bioindicators now deliver results in hours instead of days, revolutionizing safety protocols.
LISTER BIOMEDICAL CO.,LTD is a leading specialized provider of innovative sterilization monitoring solutions, dedicated to enhancing the safety and effectiveness of sterilization processes across various industries.
At LISTER, we are committed to advancing the field of sterilization by offering a comprehensive range of high-performance sterilization monitoring products. Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs),Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.Welcome to consult us!
Tags: