01 Jan, 2026
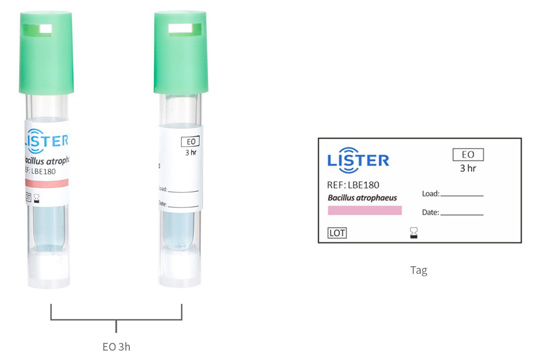
Ethylene Oxide (EO) Biological Indicators
Validating Sterilization Efficacy with Precision and Reliability
Product Overview
Our Ethylene Oxide Biological Indicators are scientifically designed to validate
the effectiveness of ethylene oxide sterilization processesin healthcare,
pharmaceutical, and medical device industries. Utilizing highly
resistant Geobacillus stearothermophilus spores, these indicators
provide a definitive measure of sterilization success, ensuring compliance with
international standards .
Key Features
Sterilization method: Ethylene oxide
Experimental bacteria: Bacillus subtilis (ATCC9372)
Bacterial content: 10^6 cfu
Reading time: 3 hours, 48 hours
Regulations: ISO13485:2016/NS-ENISO13485:2016S0 11138-1:2017:S0 11138-2:2017:1S0 11138-8:2021
Supporting monitoring equipment:Rapid Auto-Reader Incubator
User-Friendly Design
Self-contained ampoule system with integrated spore strip and growth medium.
Compatible with all standard EO sterilizers and cycle parameters.
Applications
Validation of EO sterilization for medical devices, implants, and surgical kits.
Routine monitoring of hospital central sterile supply departments (CSSD).
Quality control in pharmaceutical manufacturing and contract sterilization facilities.
For validation or bulk orders, contact our technical team at [info@listerbiomed.com] or visit [https://www.listerbiomed.com/].

Tags: