01 Jan, 2026
Introduction
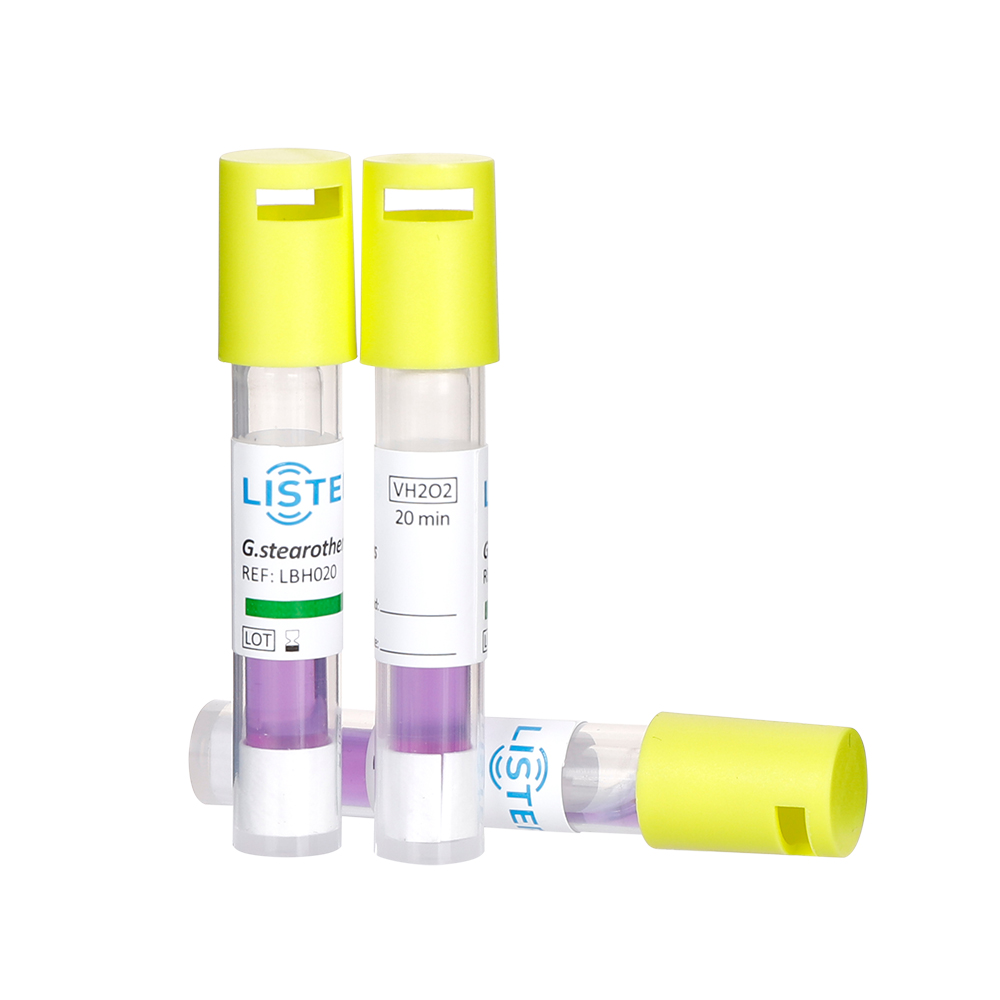
Biological indicators (BIs) play a crucial role in ensuring sterility and patient safety in the medical field. These specialized tools contain highly resistant bacterial spores that provide definitive proof of sterilization effectiveness. Hospitals, pharmaceutical companies, and medical device manufacturers rely on BIs to validate sterilization processes and comply with stringent regulatory standards.
Key Applications in the Medical Industry
1. Sterilization Validation for Medical Devices
BIs are used to confirm the efficacy of sterilization methods such as:
Steam sterilization (autoclaving) – For surgical instruments, implants, and reusable devices.
Ethylene Oxide (EtO) sterilization – For heat-sensitive devices like catheters and endoscopes.
Hydrogen Peroxide Plasma (VHP)– For delicate electronics and single-use medical tools.
They ensure that medical devices meet the Sterility Assurance Level (SAL) of 10⁻⁶, meaning a 99.9999% reduction in microbial load.

2. Monitoring Hospital Sterilization Processes
Hospitals use BIs to routinely test autoclaves and sterilizers in central sterile supply departments (CSSDs).
Regular BI testing helps detect sterilization failures before contaminated instruments reach patients, preventing surgical site infections (SSIs).
3. Pharmaceutical Manufacturing
In drug production, BIs validate sterilization of vials, syringes, and parenteral (injectable) products.
They ensure compliance with Good Manufacturing Practices (GMP) and regulatory requirements (FDA, EMA, ISO 11137 for radiation sterilization).
4. Implantable Medical Devices
BIs are critical for sterilizing pacemakers, stents, and orthopedic implants, where even minor contamination can lead to severe complications.
LISTER BIOMEDICAL CO.,LTD is a leading specialized provider of innovative sterilization monitoring solutions, dedicated to enhancing the safety and effectiveness of sterilization processes across various industries.
At LISTER, we are committed to advancing the field of sterilization by offering a comprehensive range of high-performance sterilization monitoring products. Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs), Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.
Tags: